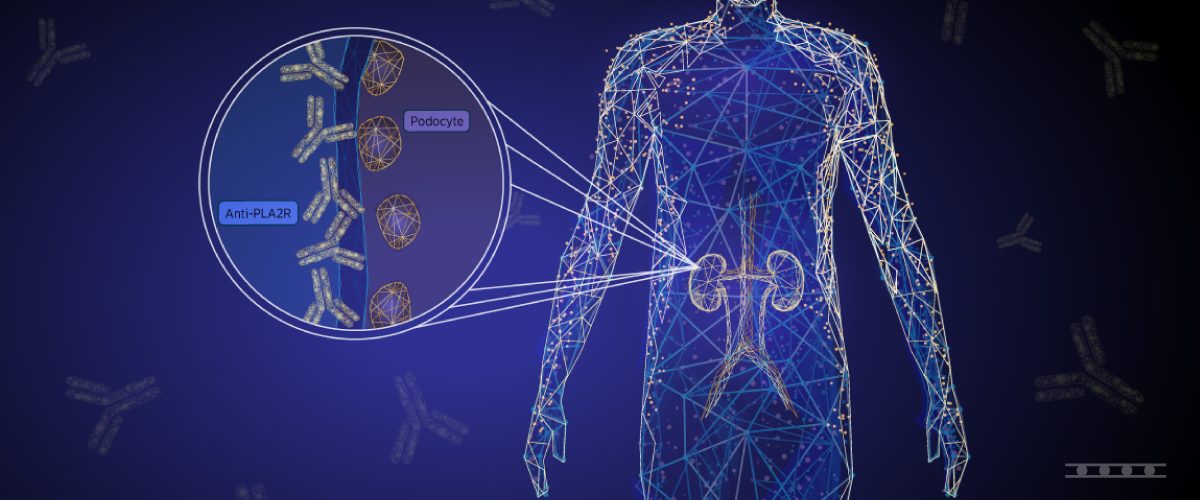
What is Membranous Nephropathy (MN)?
The kidneys are vital organs that filter out waste and toxins, and excess water from the bloodstream. In the autoimmune kidney disease Membranous Nephropathy (MN), the immune system launches an attack against the glomerulus of the kidney, which normally aids in filtering fluids and wastes. If untreated, MN leads to the buildup of toxic products, which results in progressive loss of kidney function. MN predominantly affects non-diabetic Caucasian adults above 40 years of age.1,2 and exists in two distinct forms. Primary membranous nephropathy (pMN) makes up 80% of the cases and results from the production of specific autoantibodies.3 Secondary membranous nephropathy (sMN) makes up the remaining 20% cases and is triggered by an underlying disease such as hepatitis B, systemic lupus erythematosus, or cancer, or by certain drugs.3,4
How is MN diagnosed?
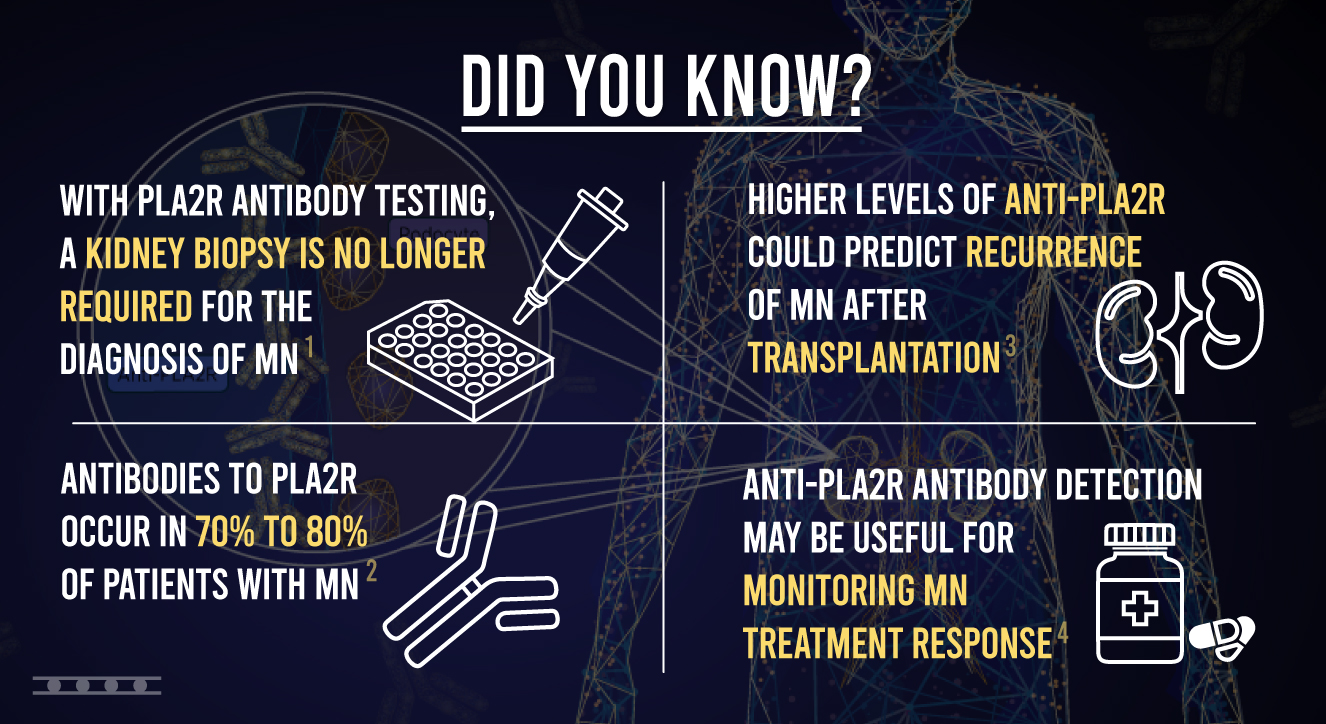
To diagnose MN, it is important to differentiate between the two forms of MN. Efforts should be taken to exclude any secondary causes of symptoms by conducting tumor screenings and distinguishing from other autoimmune diseases associated with the kidneys. Kidney biopsies have long been used for the diagnosis of glomerular disease and involves investigation of the nephrotic tissue by microscopy.5 A positive result typically reveals deposits in the kidney. Kidney biopsies have the disadvantage of being invasive, costly, and risky.5 Such risks include bruising and pain, on-going bleeding, puncture of nearby organs and infection.
A less risky, less invasive method for diagnosing MN is available with a blood test. This method involves identifying the specific autoantibodies associated with the disease. In 2009, autoantibodies against phospholipase A2 receptor (PLA2R) were discovered to be responsible for MN.6 Antibodies to PLA2R occur in 70% to 80% of patients with MN and have been shown to have a high diagnostic value.3 Due to the high occurrence of anti-PLA2R antibodies in MN and a specificity close to 100%, the detection of PLA2R antibodies have been suggested to be sufficient to confirm a diagnosis of MN in patients with NS, without the need for a biopsy.7 Accordingly, in the recent 2021 KDIGO guidelines, a practice point states that “A kidney biopsy is not required to confirm the diagnosis of membranous nephropathy (MN) in patients with nephrotic syndrome and a positive anti-PLA2R antibody test”.8
The detection of antibodies to PLA2R in patient serum can be accomplished using indirect immunofluorescence test (IIFT) and enzyme-linked immunosorbent assay (ELISA). Since the discovery of anti-PLA2R antibodies, several additional anti-podocyte antibodies have been identified in MN.9-11 Such antibodies include thrombospondin type 1 domain containing 7A (THSD7A) and Nell-1. Autoantibodies against THSDA are found in around 2.5-5% patients with MN, and often in patients that are negative for anti-PLA2R antibodies.17 EUROIMMUN holds the exclusive license to measure both anti-PLA2R and anti-THSD7a antibodies in MN.
How can antibody tests aid in MN disease and treatment monitoring?
For patients with PLA2R-associated MN, levels of PLA2Rab should be measured at 3–6-month intervals. Patients with high levels should be monitored more frequently. Persistence of PLAR2 antibodies after 3-6 months of observation is a sign of persistent disease activity, where the disappearance of PLA2R antibodies is consistent with remission.8
In MN patients, it is important to assess the risk of progressive loss of kidney function before starting immunosuppressive treatment, so that a determination can be made as to whether treatment is necessary considering adverse side effects of the therapy. High levels of PLA2R antibodies are consistent with a “high” risk of progressive loss of kidney function.8 and this risk estimation can help guide treatment options. The disappearance of anti-PLA2R antibodies precedes clinical remission and therefore can indicate that additional therapy can be avoided.8
Monitoring PLA2R antibody levels can be useful for determining therapy success. The 2021 KDIGO Guidelines state that “longitudinal monitoring of antiPLA2R antibody levels at 6 months after start of therapy may be useful for evaluating treatment response in patients with MN, and can be used to guide adjustments to therapy”.8 The persistence of anti-PLA2R antibodies at high or unchanged levels after 1 line of immunosuppressive therapy (of sufficient dose and duration) indicates resistant disease.8
The need for biomarker detection prior to kidney transplantation
For many patients with MN, renal transplantation is needed. However, the presence of anti-PLA2R antibodies poses a unique problem to renal transplant recipients. Following kidney transplantation, the risk of pMN relapse is high, occurring in up to 40% of patients.12 This risk is increased in patients having high levels of anti-PLA2R prior to transplantation.13 Therefore, the detection of anti-PLA2R prior to kidney transplantation is useful for the prediction of recurrent MN.
Anti-PLA2R antibody detection is also useful for post-transplant evaluation. According to the KDIGO guidelines, in patients with PLA2R-associated MN, “regular measurement of anti-PLA2R antibodies after kidney transplantation is advised in the first 6–12 months after transplantation.”8 The frequency of testing may vary from 1-3 months depending on pretransplant antibody status. The risk of a relapse can be predicted if titers of anti-PLA2R persist or increase.8
Conclusion
The 2009 discovery anti-PLA2R antibodies and their association with adult MN has had a huge impact on how MN patients are diagnosed and managed. Over the last 10 years, new research has demonstrated the utility of measuring anti-PLA2R antibodies and contributed to having anti-PLA2R antibodies included in 2021 KDIGO guidelines for the diagnosis, prognosis, and treatment of MN.
References
- Mastroianni-Kirsztajn G, Hornig N, Schlumberger W. Autoantibodies in Renal Diseases – Clinical Significance and Recent Developments in Serological Detection. Front Immunol. 2015;6(221):https://doi.org/10.3389/fimmu.2015.00221.
- Keri KC, Blumenthal S, Kulkarni V, Beck L, Chongkrairatanakul T. Primary membranous nephropathy: comprehensive review and historical perspective. Postgrad Med J. 2019;95(1119):23-31 https://doi.org/10.1136/postgradmedj-2018-135729.
- Couser WG. Primary Membranous Nephropathy. Clinical Journal of the American Society of Nephrology. 2017;12(6):983-997 https://doi.org/910.2215/CJN.11761116.
- Frank O’Brien M. Membranous Nephropathy. Merck Manual. Glomerular Disorders Web site. https://www.merckmanuals.com/professional/genitourinary-disorders/glomerular-disorders/membranous-nephropathy. Published 2020. Accessed April 29, 2021, 2021.
- Visconti L, Cernaro V, Ricciardi CA, et al. Renal biopsy: Still a landmark for the nephrologist. World J Nephrol. 2016;5(4):321-327.
- Beck LH, Bonegio RGB, Lambeau G, et al. M-Type Phospholipase A2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. New England Journal of Medicine. 2009;361(1):11-21 https://doi.org/10.1056/nejmoa0810457.
- Bobart SA, De Vriese AS, Pawar AS, et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney International. 2019;95(2):429-438.
- KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4s):S1-s276.
- Tomas NM, Beck LH, Meyer-Schwesinger C, et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. New England Journal of Medicine. 2014;371(24):2277-2287 https://doi.org/2210.1056/nejmoa1409354.
- Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney International. 2020;97(1):163-174 https://doi.org/110.1016/j.kint.2019.1009.1014.
- Sethi S, Madden BJ, Debiec H, et al. Exostosin 1/Exostosin 2–Associated Membranous Nephropathy. Journal of the American Society of Nephrology. 2019;30(6):1123-1136 https://doi.org/1110.1681/asn.2018080852.
- Seitz-Polski B, Lambeau G, Esnault V. Membranous nephropathy: Pathophysiology and natural history. Nephrol Ther. 2017;13 Suppl 1:S75-s81 https://doi.org/10.1016/j.nephro.2017.1001.1012.
- Stahl R, Hoxha E, Fechner K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med. 2010;363(5):496-498 https://doi.org/410.1056/nejmc1003066.