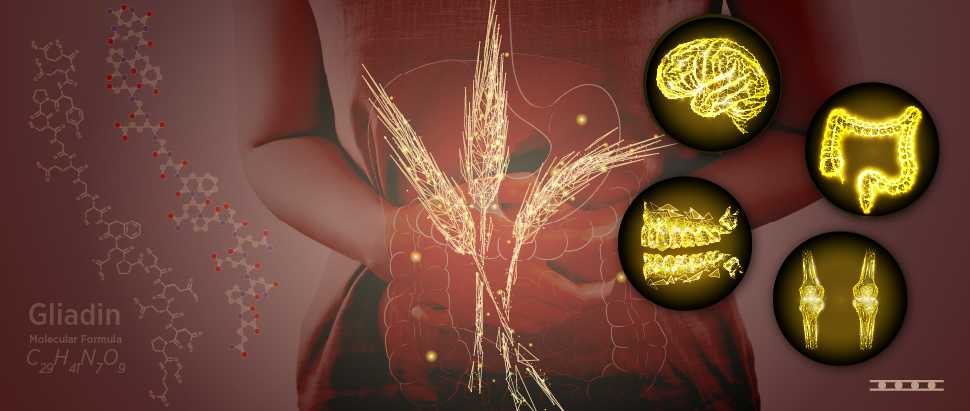
What is celiac disease?
Celiac disease (CD), also known as gluten-sensitive enteropathy, is prompted by an autoimmune reaction to the gluten protein found in wheat, rye, and barley.1 2
In people with CD, eating gluten causes an immunological reaction in the small intestine. 2Over time, this response damages the coating of the small intestine and stops it from absorbing certain nutrients (malabsorption). Diarrhea, fatigue, weight loss, bloating, and anemia are all frequent indications of intestinal damage, which can have serious repercussions. 1 2
Malabsorption can impact the growth and development of children and cause similar symptoms to those observed in adults.3
The small intestine is lined with tiny hair-like projections called villi. They include blood vessels and aid in nutrition absorption. With damaged villi, your small intestine is incapable of absorbing nutrients from food efficiently. This can ultimately lead to malnourishment, bone density loss, infertility, miscarriage, or even neurological problems or certain malignancies.3
Refractory or nonresponsive CD happens when, after at least a year of avoiding gluten, a person’s CD does not recover.
Most CD sufferers are unaware of their disorder. According to researchers, as low as 20% of people with the condition receive the correct CD diagnosis 4. Due to variable symptoms, a diagnosis of CD may take years.
Celiac illness is not synonymous with gluten intolerance or gluten sensitivity. Experiencing symptoms may cause individuals to decide to avoid gluten, even without any immunological response or damage to the small intestine.5
While there is no therapy for CD, accepting a firm gluten-free diet can help regulate symptoms and promote intestinal overhaul in most people.2
CD is thought to impact 1% of the world’s population, with females being more affected.6
Furthermore, about 2.5 million Americans remain undiagnosed in the United States, increasing their risk of having another autoimmune disorder by roughly 20 %.7
CD Pathogenesis
Gluten comprises two primary proteins, gliadin and glutenin, which are the key environmental variables known to be necessary for CD activation. Furthermore, CD is frequently seen in genetically predisposed individuals who contain particular genes called HLA-DQ2 and DQ8 (in more than 90% and 5% of instances, respectively).8
Environmental impacts include infant gastrointestinal illnesses, the amount of gluten and timing consumed around the time of stopping, and the presence or absence of nursing.9
The human upper gastrointestinal tract digests gluten poorly in CD patients. As a result, large gliadin molecules are found in the gut. Such molecules are presumably capable of entering the intestinal epithelium during gastrointestinal illnesses. The immunological response to the presence of these poisonous gliadin fragments leads to tissue damage. The tissue injury causes the pathological findings of villous atrophy and inflammation.10
To summarize, both environmental and genetic factors (HLA-DQ2/DQ8 genes) are considered necessary for the development of CD. Thus, in individuals who test negative for both DQ2 and DQ8, CD is essentially ruled out, and no additional testing is necessary.10
Individuals with CD have two distinct immunological responses in the bowel. The first is an immunological reaction in the epithelium. The epithelium is a single layer of cells on the surface of the gut that serves as a primary barrier for hazardous chemicals, preventing them from entering the intestine, and serves as a location for nutrient absorption. In CD, the epithelium is invaded by lymphocytes, and the epithelial cells are demolished. The innate immune system is the immune system support responsible for this damage.
The second immunological process in CD occurs in the lamina propria, the tissue beneath the intestine’s epithelial lining. The adaptive immune system is an element of the immune response. This process occurs in the lamina propria and is the location of gliadin interaction with tissue transglutaminase and the DQ2/DQ8 molecules, as well as the creation of cytokines that cause tissue injury and villous atrophy.
CD Diagnostics
Screening of CD is possible with a simple blood test.11When CD patients who are on a gluten-free diet consume some gluten they have higher-than-normal levels of some specific antibodies because the gluten perceives as a threat to their body. 8 2
Although CD can develop at any age, it is more common in children and usually occurs within the first two years of life. Any delay in diagnosing or treating CD might result in long-term consequences such as iron deficiency anemia, early onset osteoporosis, infertility, or gastrointestinal malignancies. Because CD is genetic, there is a 10% probability that first-degree relatives will develop it if certain HLA genes are present.12
It is recommended to do a tTG-IgA test as a first step, especially in children. The Tissue Transglutaminase IgA antibody (tTG-IgA) is a blood test that looks for antibodies or immunoglobulins, which are immune system proteins that are activated in CD patients. Celiac disease can be confirmed by a high level of antibodies in the blood. According to the American College of Gastroenterology (ACG) and European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) recommendations, immunoglobulin A (IgA) tTG is the best single test for CD identification in patients above the age of two. If IgA levels are low or not detectable, an IgG-based test (anti-DGP testing) can be used. Additionally, in some cases, HLA genotypic testing can be a useful technique of ruling out CD. 13
Genetic testing
Up to 25-30% of the general population who suffer from CD, have one or both HLA DQ2 and DQ8 genes. However, carrying HLA DQ2 and/or DQ8 is not a diagnosis of CD, nor does it guarantee that you will acquire CD in the future. Nevertheless, if you have HLA DQ2 and/or DQ8, your chance of having CD is 3% rather than 1% in the general population.14
CD is inherited, in other words- it runs in families. First-degree relatives (parents, siblings, and children) with the same genotype as the CD family member have a 40% risk of developing the disease. When the genotype is unknown, the overall chance of getting CD ranges from 7% to 20%.13
The risk of CD is minimal if an individual does not have the DQ2 and DQ8 alleles. Because of the lack of uniformity and the high cost of genotypic testing, alternative quick and cost-effective tests are critical for providing new insights into the value of HLA typing in the diagnosis and management of CD.
Endomysial antibody (EmA)-IgA testing
Although not as sensitive as the tTG-IgA test, the EmA test has a specificity of about 100%, making it the most specific test for CD.6 8 Approximately 5-10% of CD patients do not have a positive EmA test. It is normally reserved for individuals who are difficult to diagnose and for children to avoid biopsy. EUROIMMUN’s EmA IFA method is one of the most reliable ones to diagnose the CD without biopsy.
Anti-Gliadin (GAF-3X)- IgA and IgG testing
Other serological tests available are the anti-gliadin (GAF-3X) (IgA, IgG) ELISA, and IIFT. For these tests, EUROIMMUN developed a designer antigen, called GAF-3X, which is a special protein used to best stimulate a host immune response. GAF-3X antibodies have a very high sensitivity and specificity, particularly for IgG. The novel EUROPLUS Anti-Gliadin (GAF-3X) IIFT can therefore identify CD patients with specific IgA deficiency. Only around 5% of patients do not have IgA antibodies. As a result, an accurate IgG test is critical.15
The simultaneous detection of anti-GAF-3X IgG and anti-tTG IgA antibodies results in maximum diagnostic accuracy. These serological tests can be used for monitoring CD patients.
Takeaways
CD is an autoimmune disease. It might occur at an early age or later in life. The traditional method for diagnosing CD is a biopsy, but there are now several different serological tests available. tTG IgA is a specific antigen that is activated by the body in CD patients. The combined detection of anti-GAF-3X IgG, and anti-tTG IgA antibodies yields the most accurate diagnostic results. The genetic test (HLA DQ2/DQ8) can be done to rule out CD.
Patients with CD should be on a gluten-free diet and need to be monitored regularly with a blood test. EUROIMMUN offers different portfolios for CD diagnosis and monitoring: ELISA (Anti tTG, glidian-GAF-3X, IgA, and IgG), IIFT (Tissue, EUROPLUS, IgA, and IgG), EUROLINE (IgA and IgG), and EUROARRAY (HLA DQ2/DQ8).
References
- Celiac disease – Symptoms and causes – Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/celiac-disease/symptoms-causes/syc-20352220.
- Celiac Disease: Symptoms, Causes, Diagnosis, Treatment, Risk Factors. https://www.webmd.com/digestive-disorders/celiac-disease/celiac-disease.
- Zuvarox, T. & Belletieri, C. Malabsorption Syndromes. StatPearls [Internet] (StatPearls Publishing, 2021).
- Celiac Disease. WebMD https://www.webmd.com/digestive-disorders/celiac-disease/celiac-disease.
- Celiac Disease Testing and Diagnosis | BeyondCeliac.org. https://www.beyondceliac.org/celiac-disease/get-tested/.
- Mahadov, S. & Green, P. H. R. Celiac Disease. Gastroenterol. Hepatol. 7, 554–556 (2011).
- What is Celiac Disease? Celiac Disease Foundation https://celiac.org/about-celiac-disease/what-is-celiac-disease/.
- The prevalence, pathogenesis and diagnostics of celiac disease | Medical Laboratory Observer. https://www.mlo-online.com/disease/article/21115326/the-prevalence-pathogenesis-and-diagnostics-of-celiac-disease.
- Lionetti, E. & Catassi, C. The Role of Environmental Factors in the Development of Celiac Disease: What Is New? Diseases 3, 282–293 (2015).
- Pathogenesis. Celiac Disease Center at Columbia University Medical Center https://celiacdiseasecenter.columbia.edu/celiac-disease/pathogenesis/.
- Aug 10, Allergy, 2021 | & Autoimmune |. Beyond the Biopsy: Serological Testing Offers Greater Diagnosis Efficacy and Monitoring of Celiac Disease. Clinical Lab Products https://clpmag.com/disease-states/allergy-autoimmune/beyond-the-biopsy-serological-testing-offers-greater-diagnosis-efficacy-and-monitoring-of-celiac-disease/ (2021).
- Uenishi, R. H. et al. Screening for celiac disease in 1st degree relatives: a 10-year follow-up study. BMC Gastroenterol. 14, 1–5 (2014).
- Martina, S. et al. Genetic susceptibilty and celiac disease: what role do HLA haplotypes play? Acta Bio Medica Atenei Parm. 89, 17 (2018).
- CECILIO, L. A. & BONATTO, M. W. THE PREVALENCE OF HLA DQ2 AND DQ8 IN PATIENTS WITH CELIAC DISEASE, IN FAMILY AND IN GENERAL POPULATION. Arq. Bras. Cir. Dig. ABCD Braz. Arch. Dig. Surg. 28, 183–185 (2015).
- Tests in Coeliac: tTG & GAF-3X (IgA, IgG) | Vinmec. https://www.vinmec.com/en/news/health-news/general-health-check/tests-in-coeliac-ttg-gaf-3x-iga-igg/.