Less than a year after the World Health Organization (WHO) declared COVID-19 a global pandemic, the authorization of several novel COVID-19 vaccines has created hope for a healthier future. However, the recent rise of new SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) variants has made the task of deciphering the immune response to COVID-19 ever more pressing. Recent studies have demonstrated that both humoral and cellular immunity play a role in protection from COVID-19.1,2 Additionally, the development of novel COVID-19 vaccines requires a look at the immune response in order to determine the effectiveness of the vaccine at preventing infection.
The Immune System
When the body encounters a pathogen, either a virus, bacterium, fungus, or parasite, it launches an attack against the pathogen to protect the body. This attack is called an immune response. During this attack, the immune system recognizes certain parts of the invading pathogen, known as the antigen. So, what happens next? The immune system launches a cascade of attacks against the pathogen. There are two mechanisms by which this happens:
Humoral immunity is a process by which certain cells, called B cells, create ammunition that can specifically recognize the antigens. These antigen recognizing proteins are called antibodies. When an antibody binds to an antigen, few things can happen. Firstly, the binding of the antibody to the antigen can disarm and block the pathogen from launching its attack. Secondly, the antigen-bound antibody calls for help from other immune cells to destroy the pathogen. Antibodies can recognize an antigen and bind to it, but they can’t destroy the virus without help. By binding to the pathogen, antibodies work like a tag to allow other immune cells to recognize the pathogen, and subsequently eliminate it.
When the body is infected by a pathogen, certain cells of the immune system decide to devour the pathogen like a tasty snack. These prideful immune cells then want to show off what they just ate, so they put the specific antigen belonging to the pathogen on their outer surface for all to see. This boastful act sparks the interest of another set of immune cells called T cells, which, in turn can kill the invading pathogen. This process is referred to as cell-mediated immunity. T cells can also destroy host cells that have been infected and can help direct other immune cells to do their jobs of attacking the pathogen. For example, certain T cells can communicate with B cells to tell them to start producing antibodies. In this way the humoral immune system and cell-mediated immune system are connected.
Immune Cells and Long-term Protection
Similar to other viral infections, when a person is infected with SARS-CoV-2 virus, B cells and T cells get to work in a matter of days to weeks to defend the body from the viral infection.3,4 (Figure 1) Importantly, the job of B cells and T cells doesn’t end when symptoms of the disease are over, as these cells help retain a memory of the infection. When B cells and T cells multiply during an active infection, some of the cells are stored so that the body can be prepared for a future attack.5 Therefore, upon a second exposure to SARS-CoV-2 virus, there is a quicker and stronger response to the virus compared to the response from the first exposure, thanks to these memory cells.6 This process is known as protective immunity. In terms of COVID-19, there is still uncertainty about whether antibodies and T cells can provide protective immunity to the SARS-CoV-2 virus, and for how long that immunity might last. Recent efforts have identified cases where memory B cells and virus-specific antibodies could be detected in the blood of a recovered patient for as long as 8 months, offering possible protection from a repeat infection.7,8
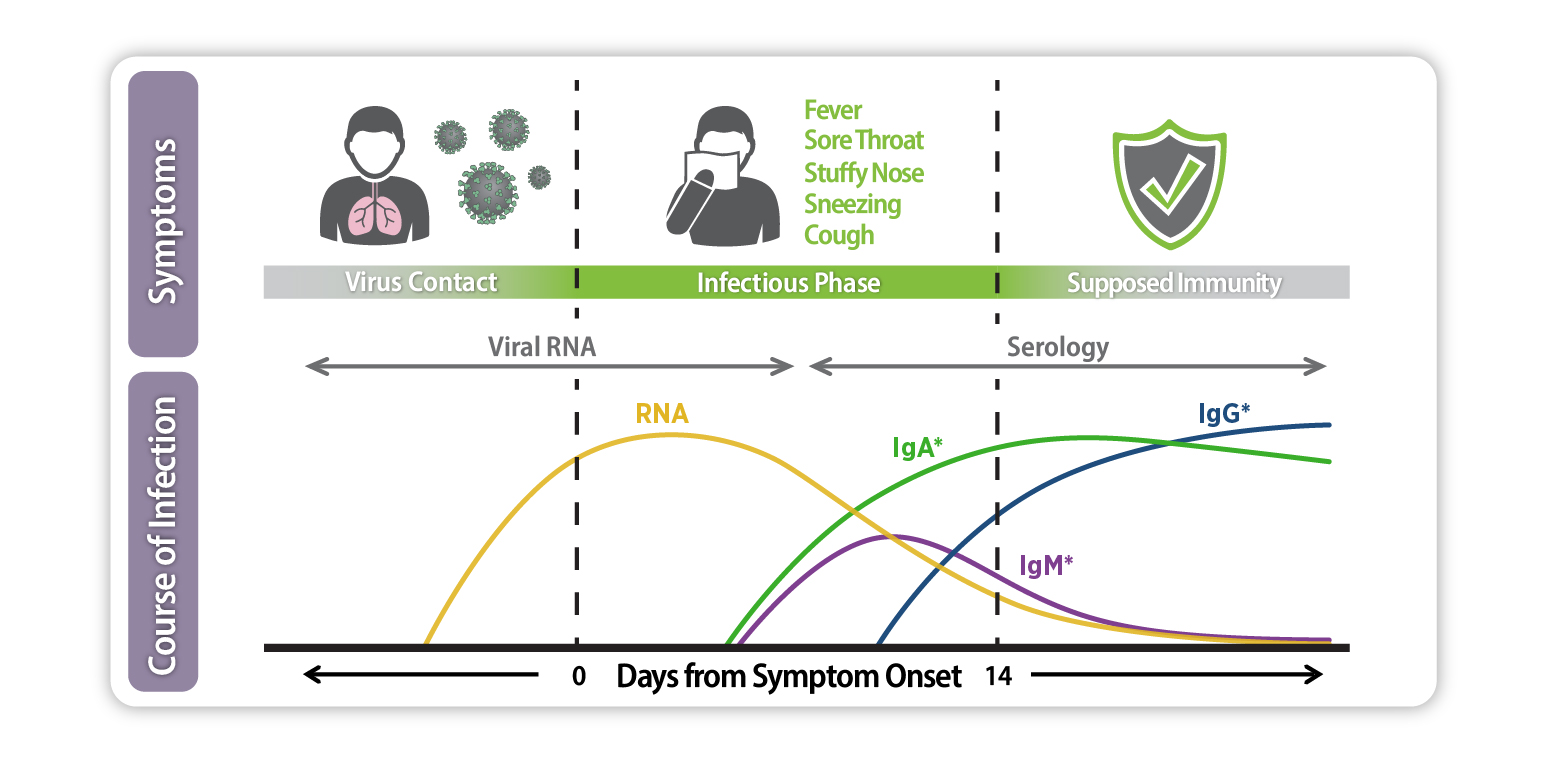
Figure 1. Serological response to SARS-CoV-2 infection. After a person comes in contact with SARS-CoV-2, antibodies (IgA, IgM and IgG) are produced at different time points. Levels of antibodies remain in the body even after symptoms of the disease subside, providing potential protection from a repeat infection.
Vaccines: Tricking the Immune System
Why are vaccines critical in a viral infection? Vaccines prepare your immune system to fight a potential infection by taking advantage of the fact that the immune system has a memory. Vaccines work by tricking the immune system into thinking that it is under attack by the foreign pathogen. While many different forms of vaccines exist, they all ultimately work the same way, by introducing the virus-specific antigen into the body.9 Then, the immune system can get to work and also retain a memory of the encounter, as described earlier. As of April 2021, three vaccines have been authorized for emergency use for the prevention of COVID-19 in the United States. The Moderna and Pfizer-BioNTech COVID-19 vaccines are messenger Ribonucleic Acid (mRNA)-based vaccines. mRNA carries instructions for building proteins inside of our cells. More specifically, the mRNA in the Moderna and Pfizer-BioNTech vaccines carry the instructions on how to make a particular SARS-CoV-2 antigen called the spike protein. The spike protein is located on the surface of the SARS-CoV-2 virus and aids in the attachment of the virus to human cells during infection. (Figure 2) This protein alone is harmless but is effective at tricking the immune system to activate an army of immune cells against this part of the virus. When mRNA is injected into our bodies it needs to enter our cells in order to be made into protein. Since mRNA is fragile, and breaks down quickly, scientists wrap the mRNA in a protective bubble called a lipid nanoparticle. The recently authorized Janssen COVID-19 vaccine functions in a similar way to the mRNA vaccines. The difference is that the Janssen vaccine contains DNA, which is converted into the mRNA in our cells. Then the mRNA carries the instructions for our cells to build the spike protein. Whereas, the direct mRNA vaccines (Pfizer-BioNTech and Moderna) immediately directs the cells to produce the antigen. Unlike the mRNA vaccines which use a lipid nanoparticle for protection, the Janssen vaccine puts the DNA into a non-infectious virus to protect it from breakdown. All three of these vaccines have been found to be effective against moderate to severe SARS-CoV-2 infections and hospitalization.10 Recently, administration of the Jansen vaccine has been put on hold due to a small number of reported cases (6 cases) of a rare blood clot in people who received the Janssen vaccine. It is not yet been shown whether the blood clots are related to the vaccine.

Figure 2. Schematic representation of the SARS-CoV-2 structure. The spike (S) protein enables host cell attachment and fusion. The nucleocapsid (N) protein is responsible for packaging the viral genome RNA and for viral assembly.
Studies have shown that vaccines induce the production of antibodies both capable of blocking the SARS-CoV-2 virus from launching an attack on our cells, and able to recognize the spike protein antigen, facilitating the destruction of the pathogen as described previously.11,12 COVID-19 vaccines have also been shown to induce the production of T cells that can directly destroy the virus.11,12 Such a cell-mediated immune response is important because T cells could be more effective against the emerging SARS-CoV-2 variants than antibodies.13,14 It is important to note that the new variants feature small singular changes (called point mutations) in the spike protein which, for now, are still recognized by the humoral and cellular immune responses mediated by vaccination. Recent data has revealed that the Pfizer-BioNTech and Moderna vaccines continue to be effective against COVID-19 for at least six months.15,16
Perspective
The detection and monitoring of an individual’s immune response to both natural COVID-19 infection and vaccination is crucial for the purposes of assessing viral spread in a community, assessing vaccine effectiveness, and for the determination of a person’s immune response in order to provide an understanding of their long-term immunity.17 However, further research is still needed to understand exactly how the level of antibodies and T cells correlates with the degree and duration of immunity. Currently, tests are available for the identification potential donors of antibody containing plasma for the treatment of patients with an active SARS-CoV-2 infection.18 With the emergence on new SARS-CoV-2 variants that feature mutations in the spike protein, a focus on cell-mediated immunity is of utmost importance.
References
- Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584(7819):115-119.
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489-1501.e1415.
- Vibhuti Kumar Shah PF, Aftab Alam, Dipyaman Ganguly, Samit Chattopadhyay. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Frontiers in Immunology. 2020.
- Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52(6):971-977.e973.
- Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063.
- Rodda LB, Netland J, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Res Sq. 2020:rs.3.rs-57112.
- Choe PG, Kim K-H, Kang CK, et al. Antibody Responses 8 Months after Asymptomatic or Mild SARS-CoV-2 Infection. Emerging Infectious Disease journal. 2021;27(3):928.
- Hartley GE, Edwards ESJ, Aui PM, et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Science Immunology. 2020;5(54):eabf8891.
- Callaway E. The race for coronavirus vaccines: a graphical guide. Nature Publishing Group. Nature Web site. https://www.nature.com/articles/d41586-020-01221-y. Published 2020. Updated 2020-04-28. Accessed 7805, 580.
- Brueck H. CDC: Real-world data shows Pfizer’s and Moderna’s COVID-19 vaccines are 90% effective at preventing infection. 2021.
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. New England Journal of Medicine. 2020;383(20):1920-1931.
- Sahin U, Muik A, Vogler I, et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020:2020.2012.2009.20245175.
- Donal TS, Adam CH, Javier G-J, et al. Vaccine-induced immunity provides more robust heterotypic immunity than natural infection to emerging SARS-CoV-2 variants of concern. Res Sq. 2021.
- Tarke A, Sidney J, Kidd CK, et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep Med. 2021;2(2):100204.
- Pfizer and BioNTech Confirm High Efficacy and No Serious Safety Concerns Through Up to Six Months Following Second Dose in Updated Topline Analysis of Landmark COVID-19 Vaccine Study [press release]. 2021-4-1 2021.
- Moderna Highlights Publication of Antibody Persistence Data of its COVID-19 Vaccine out to 6 Months in the New England Journal of Medicine [press release]. 2021-4-7 2021.
- A Closer Look at COVID-19 Diagnostic Testing | FDA. @US_FDA. https://www.fda.gov/health-professionals/closer-look-covid-19-diagnostic-testing. Published 2021. Accessed.
- Convalescent Plasma. IDSAInfo. https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/convalescent-plasma/. Published 2021. Accessed.
Relevant links
https://www.coronavirus-diagnostics.com/
https://www.euroimmun.com/products/infection-diagnostics/id/sars-cov-2-infection-covid-19/



Very well written. Clear and concise.
Hi, I have a question because while i am reading ,said if you got a vaccinated already either moderna or pfizer the effectivity will be until six months only.
My question is ….
What happened in six months after you got a vaccine?
The vaccine will no longer effective already to protect our body or can re produce an antibody from the vaccine itself?
Thanks for sharing and the explanation please update us if possible send for me an email so that we can get more informations regarding for anything new for COVID-19 situation.
Hoping for more information.
Warmest Regards,
Mercy
Hi Mercy,
Thank you for your question. While you are still protected from the virus 6 months after vaccination, the efficacy of the vaccine decreases. For example, very recent data has shown that the effectiveness of the Pfizer COVID-19 vaccine falls from 96% to 84% 6 months after the second dose. Vaccine manufactures are therefore looking into booster shots which can allow the body to produce more antibodies.
Best,
Ilana