SARS-CoV-2, commonly known as the coronavirus disease 2019 (COVID-19) poses an unprecedented public health crisis. While scientists and clinicians worldwide are still working to understand the virus, recent studies show possible onset of autoimmune diseases following COVID-19 infection.
Viral Pathogens Can Trigger Autoimmune Response
Generally, the immune system is able to differentiate self-components from foreign pathogens. However, when the immune system fails to distinguish these structures, autoantibodies are produced causing an inflammatory response leading to autoimmune diseases. One of the most common triggers of autoimmunity are viral pathogens.1 Viral pathogens may promote autoantibody and cytokine production through activation of non-specific B and T lymphocytes.1
With an increase in coronavirus cases, particularly in immunocompromised individuals, it is evident that there may be an association between SARS-CoV-2 and autoimmune diseases.2 Comorbidities that put patients at high-risk of severe SARS-CoV-2 infection include cancer, chronic kidney disease, chronic obstructive pulmonary disorder, autoimmune myocarditis, multiple sclerosis, systemic lupus erythematosus (SLE), and rheumatoid arthritis, among others.3 In addition to preexisting conditions, autoimmune responses may contribute to the onset of severe illness due to SARS-CoV-2 infection. Although the etiology of many autoimmune diseases is not entirely understood, some aspects such as environmental triggers (i.e., bacterial/viral infections), physical factors, or genetic factors may contribute to the severity of SARS-CoV-2.4 A new study has determined the role of SARS-CoV-2 in triggering an autoimmune response in patients with no prior history of autoimmune diseases.1
Anti-Nuclear Antibodies found in Majority of SARS-CoV-2 patients
A study by Sacchi et al.1 details whether SARS-CoV-2 infection could induce an autoimmune response through the production of autoantibodies. In a recent study, 80 adult patients (40 with confirmed COVID-19 diagnosis, 40 healthy blood donors as controls) with no previous clinical history of autoimmune diseases were analyzed for various autoantibodies. The antibodies targeted in this study included antiphospholipid antibodies (anti-cardiolipin, anti-beta2-glycoprotein IgA and IgG), and anti-Saccharomyces cerevisiae antibodies (ASCA) IgA and IgG, Anti-neutrophil cytoplasmic antibodies (ANCA) and anti-nuclear antibodies (ANA).1
Like other viral infections, SARS-CoV-2 caused an elevated inflammatory response in patients. Increased production of inflammatory cytokines can aid in fighting the virus or can aggravate inflammatory chemokines resulting in “inflammatory storm” that worsen patients’ conditions. COVID-19 patients with higher levels of IL-6 also had the most severe clinical consequences.1 The connection found between SARS-CoV-2 infection and inflammatory responses reinforces the idea that upsurges in IL-6 levels from baseline can be useful in detecting progression of early SARS-CoV-2 infection to more complex disease.1,6
A strong inflammatory response can also trigger a harmful autoimmune response. In the study by Sacchi et al., patients with no previous clinical record of autoimmunity became positive for various autoimmune markers following SARS-CoV-2 infection.1 A majority of the patients (57.5%) were positive for anti-nuclear antibodies (ANA).1 ANA is typically an important biomarker in diagnosing ANA-associated rheumatic diseases (AARD) such as SLE, Scleroderma, Sjogren’s syndrome, etc. In general, ANAs are observed in 25% of healthy subjects, but in this study, the ANA positive rate was two-fold higher compared with what is described with the healthy population.1 Additionally, 25% patients were positive for ANCA and similar rate for ASCA (Figure 1). ANCA attacks neutrophils leading to an autoimmune condition called vasculitis resulting in inflammation and swelling of the blood vessels.7 ASCA may help differentiate Crohn’s disease from ulcerative colitis. 8,9
Overall, ANA, ANCA, and ASCA IgA antibodies were significantly prevalent in COVID-19 patients.1 Interestingly, ANCA may play a role not only in autoimmunity, but also in patient survival.1 The percentage of deceased patients was slightly higher for ANCA(+) patients (40%) compared to those with ANA (39.13%) or ASCA (30%).1 All the ANCA(+) patients who expired during the study showed an X-ANCA immunofluorescence pattern, eluding to the fact that they reacted with neutrophil antigens other than proteinase 3 and myeloperoxidase.10 Thus, there may be an association between X-ANCA positivity and severity of SARS-CoV-2 disease resulting in death.1 Based on these findings, COVID-19 patients who also possessed ANA and ANCA autoantibodies had the worst clinical consequences ultimately leading to death.1
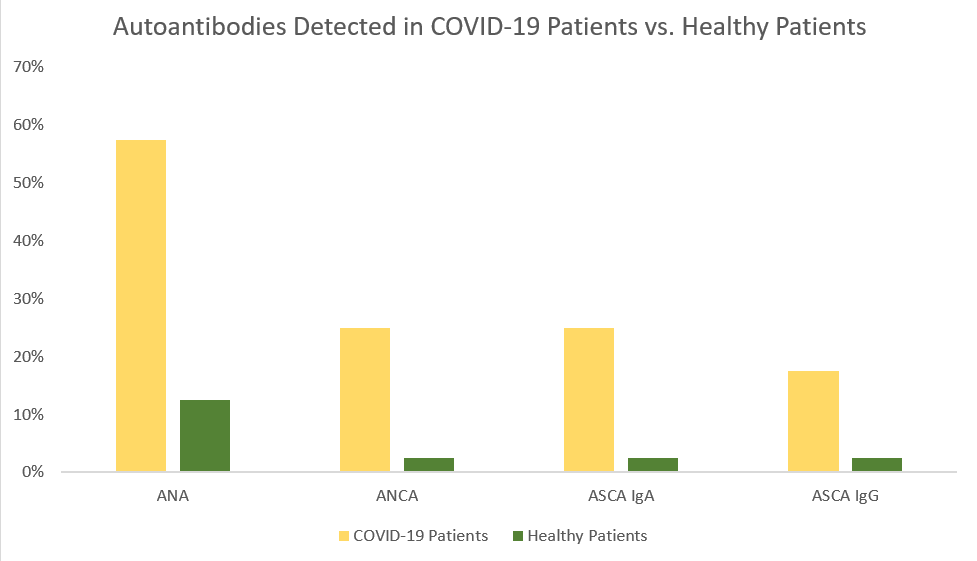
Figure 1. Summary of the presence of specific autoantibodies in infected (COVID-19(+)) and healthy (COVID-19(-)) patients. Each group had 40 subjects. Positive refers to the presence of the autoantibody analyzed. Out of the healthy population, only 5% presented with these autoantibodies while over 30% of the COVID-19(+) population presented with positive autoantibodies. This table was created using data directly taken sourced from Sacchi et al.1
Assessing Autoimmunity Markers in SARS-CoV-2 Patients
Despite the limitation with the sample size, it is firmly demonstrated that COVID-19 is associated with autoimmunity, particularly the development of key autoantibodies such as ANA, ASCA, and ANCA.1 Autoimmune response could elucidate wide variability in recovery times and symptom improvement despite the attenuation of SARS-CoV-2 virus. COVID-19 patients already have a diminished respiratory system, which can be further negatively affected by autoimmunity.1
It is imperative to consider the impact of autoimmunity on COVID-19 patients not only to choose the right therapies but to also determine if plasma transfer is a viable option. COVID-19 patients have strong autoantibody positivity (i.e., ANA, ASCA, and ANCA).1 These autoantibodies are not only used as disease markers but could also be pathogenic. Therefore, performing transfusions or transferring antibodies using these neutralizing autoantibodies may not be the most optimal course of action for these patients.
Further studies are needed to better understand if there is correlation between COVID-19 disease stage and the autoimmunity response. Additionally, it is vital to understand if these autoimmune responses are only transitory or might persist longer, resulting in chronic autoimmune conditions.
References
- Maria C. Sacchi ST, Paolo Stobbione, Lisa Agatea, Piera De Gaspari, Anna Stecca, Ernesto C. Lauritano, Annalisa Roveta, Renato Tozzoli, Roberto Guaschino, Ramona Bonometti. SARS-CoV-2 INFECTION AS A TRIGGER OF AUTOIMMUNE RESPONSE. American Society of Clinical Pharmacology & Therapeutics. 2020.
- Institute GA. COVID-19 & AUTOIMMUNE DISEASE: WHAT WE KNOW NOW. https://www.autoimmuneinstitute.org/covid-19-autoimmune-disease-what-we-know-now/. Published 2020. Accessed.
- Prevention CfDCa. People with Certain Medical Conditions. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Published 2021. Accessed.
- Michael Ehrenfeld AT, Laura Andreoli, Marco Cattalini, Assaf Greenbaum, Darja Kanduc, Jaume Alijotas-Reig, Vsevolod Zinserling, Natalia Semenova, Howard Amital, and Yehuda Shoenfeld. Covid-19 and autoimmunity. Autoimmunity Reviews. 2020.
- Yan Zhang MX, Shulan Zhang, Peng Xia, Wei Cao, Wei Jiang, Huan Chen, Xin Ding, Hua Zhao, Hongmin Zhang, Chunyao Wang, Jing Zhao, Xuefeng Sun, Ran Tian, Wei Wu, Dong Wu, Jie Ma, Yu Chen, Dong Zhang, Jing Xie, Xiaowei Yan, Xiang Zhou, Zhengyin Liu, Jinglan Wang, Bin Du, Yan Qin, Peng Gao, Xuzhen Qin, Yingchun Xu, Wen Zhang, Taisheng Li, Fengchun Zhang, Yongqiang Zhao, Yongzhe Li, Shuyang Zhang. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. New England Journal of Medicine: Peking Union Medical College Hospital;2020.
- Eric A. Coomes HH. Interleukin-6 in Covid-19: A systematic review and metaanalysis. Reviews in Medical Virology. 2020.
- Max Yates RW. ANCA-associated vasculitis. Clinical Medicine. 2017.
- L. J. WALKER MCA, H. E. DRUMMOND, B. R. K. SMITH, E. R. NIMMO, I. D. R. ARNOTT, J. SATSANGI. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn’s disease are associated with disease severity but not NOD2/CARD15 mutations. Clinical and Experimental Immunology. 2004.
- Seibold F. ASCA: genetic marker, predictor of disease, or marker of a response to an environmental antigen? Gut. 2005.
- Ming-Hui Zhao CML. Antineutrophil Cytoplasmic Autoantibodies with Specificity Other Than PR3 and MPO (X-ANCA). In: Shoenfeld JBPaY, ed. Autoantibodies. ScienceDirect1996.