The recent study conducted by De Meyer et al. demonstrated that EUROIMMUN ELISA and SIMOA (single molecule array) platform detected cerebral amyloidosis with equivalent accuracy through measuring the plasma Aβ42/Aβ40 ratio.
Hallmarks of Alzheimer’s Disease
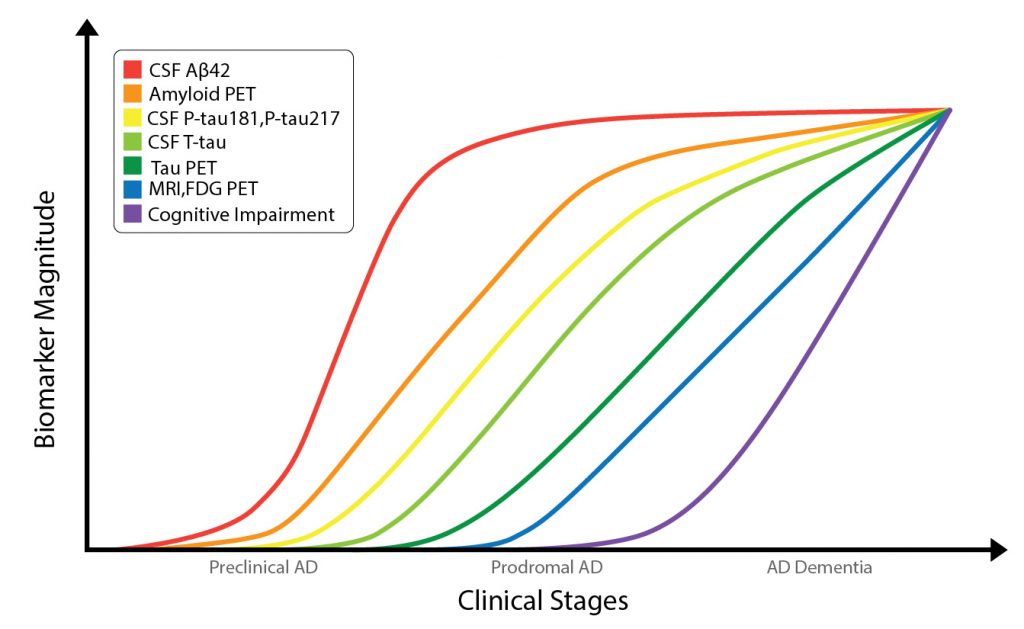
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly and is observed in almost 70% of all dementia cases. The Alzheimer’s Association estimates that 5.8 million Americans aged 65 years and older are living with AD and other dementias and that number is projected to quadruple by 2050.1 The neuropathological hallmarks include the accumulation of beta-amyloid (Aβ) plaques and tau tangles together with other neural alterations in the brain parenchyma.2,3 It is hypothesized that these neuropathological changes in AD begin approximately 20 years before the appearance of cognitive symptoms (Figure 1).4,5 Currently, the gold standard for the definitive detection of amyloid-pathology in a patient’s brain is amyloid staining in postmortem autopsy brain tissue.5 Therefore, there is an urgent need for pre-mortem biomarkers that demonstrate strong indications of amyloid pathology. Currently, a probable diagnosis is typically based on clinical evaluation, analyses of these hallmark biomarkers in cerebrospinal fluid (CSF) and through imaging.2,6
Comparison of ELISA and SIMOA Technologies
De Meyer et al.8 performed a prospective, cross-sectional study to compare the assay performances of a commercially available ELISA kit (EUROIMMUN, Germany) with the prototype SIMOA Amyblood assay (ADx Neurosciences and UMC Amsterdam) for measuring the plasma Aβ1-42/Aβ1-40 ratio to detect early cerebral amyloidosis in a non-demented elderly cohort. This study included 161 cognitively normal (CN) participants and 38 patients with amnestic mild cognitive impairment (aMCI) (total n = 199 non-dementia patients), without any major differences in age or sex.
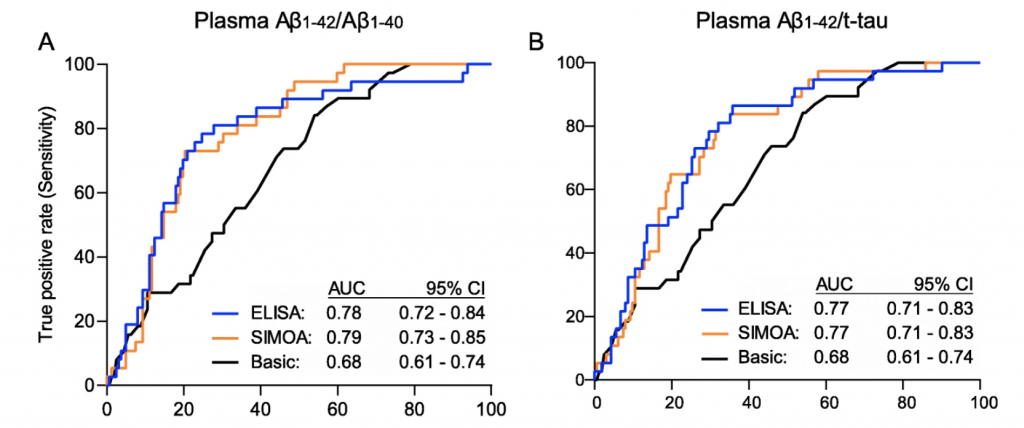
The discriminative performance of the tests to detect cerebral amyloidosis, based on amyloid-Positron emission tomography (PET), as measured by area under the ROC curve did not vary between ELISA plasma Aβ1-42/Aβ1-40 and the SIMOA platform in the entire study population (p = 0.85, Figure 2). Additionally, comparable results were observed between ELISA and SIMOA platforms in terms of sensitivity, specificity, positive and negative predictive value. Higher negative predictive value in such plasma tests are of utmost importance in order to reduce the number of patients undergoing more expensive and/or invasive confirmatory tests. The negative predictive values were ≥ 88% in both platforms. Furthermore, the performance of a basic demographic model with age and APOE status, improved with the inclusion of plasma Aβ1-42/Aβ1-40 from either platform to detect amyloid-PET positivity in the entire population. Similar results were also observed when plasma Aβ1-42/total-tau was measured using either platform (Figure 2).
This study also determined the correlations between the plasma biomarkers from both platforms and amyloid-PET and CSF Aβ1-42/total-tau. Both ELISA and SIMOA platforms showed an inverse relationship between plasma Aβ1-42/Aβ1-40 and amyloid-PET binding. As plasma Aβ1-42/Aβ1-40 decreased, amyloid-PET binding increased. In a smaller subset of patients (N=56), decreased ELISA levels of plasma Aβ1-42/Aβ1-40 aligned with decreased CSF Aβ1-42/total-tau levels in all cohorts, except the CN subgroup. These correlations were more prominent with EUROIMMUN’s ELISA technology compared to that observed with SIMOA.
Blood Tests for Detecting Cerebral Amyloidosis
In this study, EUROIMMUN ELISA and the SIMOA Amyblood assays demonstrated comparable results in detecting amyloid-PET positivity in nondemented elderly population by measuring plasma Aβ1-42/Aβ1-40. Both ELISA and SIMOA systems have the potential to considerably and equivalently reduce the number of amyloid-PET scans needed for recruiting healthy participants in clinical trials. Interestingly, the PET reduction rates are slightly higher with ELISAs compared with the SIMOA for plasma Aβ1-42/Aβ1-40 (63% versus 58%). In short, the SIMOA Amyblood Assay did not provide additional benefits to EUROIMMUN’s commercially available ELISA tests for measuring plasma amyloids. Currently, ELISA assays are much more widely available and better understood than SIMOA technology. As the more cost-efficient option compared to SIMOA, the ELISA platform can measure various AD biomarkers, support physicians in reducing inaccurate screening rates and aid in earlier detection of AD.
Overall, this study proves that the clinical performance of ELISAs have improved and can be considered comparable to the ultrasensitive SIMOA. Both methods show identical performance in detecting cerebral amyloidosis by measuring plasma ratios and would be valuable tools in clinical research settings
EUROIMMUN offers a broad portfolio of reliable, cost-effective ELISA test systems for measuring various CSF as well as plasma biomarkers in neurodegeneration. Please visit our website for more information.
References
- Alzheimer’s Disease Facts and Figures. https://www.alz.org/alzheimers-dementia/facts-figures. Published 2020. Accessed 01/09/2021, 2021.
- Khoonsari PE, Shevchenko G, Herman S, et al. Improved Differential Diagnosis of Alzheimer’s Disease by Integrating ELISA and Mass Spectrometry-Based Cerebrospinal Fluid Biomarkers. Journal of Alzheimer’s disease : JAD. 2019;67(2):639-651.
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2011;1(1):a006189-a006189.
- Jack CR, Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology. 2013;12(2):207-216.
- Sutphen CL, Fagan AM, Holtzman DM. Progress update: fluid and imaging biomarkers in Alzheimer’s disease. Biol Psychiatry. 2014;75(7):520-526.
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal Fluid tau/β-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults. Archives of Neurology. 2007;64(3):343-349.
- Mattsson-Carlgren N, Andersson E, Janelidze S, et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer’s disease. Science advances. 2020;6(16):eaaz2387-eaaz2387.
- De Meyer S, Schaeverbeke JM, Verberk IMW, et al. Comparison of ELISA- and SIMOA-based quantification of plasma Aβ ratios for early detection of cerebral amyloidosis. Alzheimer’s Research & Therapy. 2020;12(1):162.
Related Links
• EUROIMMUN Neurodegeneration ELISA Tests
• EUROIMMUN Tests For Alzheimer’s Disease
• Biocompare: EUROIMMUN Neurodegeneration Tests
• EUROIMMUN Random Access Testing Solutions for Neurodegeneration
• Article: Neurodegeneration biomarkers
• Article: New biofluid biomarkers in neurodegeneration