More than 100 biological drugs have been approved by the Food and Drug Administration (FDA) in the last half-century.1 Biologics are products derived from: living organisms comprised of sugars, proteins, nucleic acids, or complex combinations of these substances; cells; or tissues, rather than being synthesized in a laboratory.2 Biologics include antibodies to tumor necrosis factor alpha (TNF-α) such as Etanercept (Enbrel®), Infliximab (Remicade®), and Adalimumab (Humira®). Therapeutic anti-TNF-α drugs are used to treat a variety of chronic diseases, including Crohn’s Disease and ulcerative colitis.3 Unfortunately, 20-30% of patients do not respond to anti-TNF-α treatment.4 Fifty percent of patients with inflammatory bowel disease experience a relapse in disease activity during maintenance therapy.5,6
Patients should be tested for active/latent tuberculosis and viral hepatitis B and C prior to initiation of anti-TNF-α biotherapy, and be monitored for signs of infection before, during, and after treatment.7,8 Additionally, complete blood counts and liver function tests are necessary at baseline and should be monitored closely.
All biotherapies are immunogenic and trigger the production of anti-drug antibodies (ADA), which reduce drug effectiveness. Proper dosing at correct intervals could limit the production of ADA and extend the durability of the drug. The frequency of ADA incidence varies according to the drug and dose administered, the treatment scheme, the immunogenicity of each drug, and the individual’s pharmacokinetic variability.
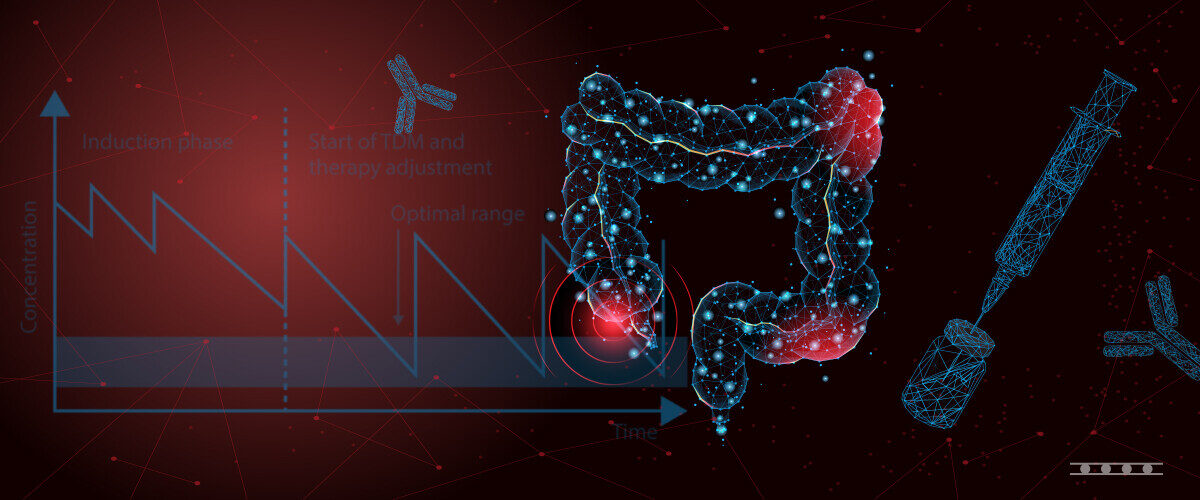
The use of Therapeutic Drug Monitoring (TDM) may provide valuable information to reduce the incidence of adverse events resulting from dosages being too high or immunogenicity of the drug. TDM measures concentrations of certain medications to determine if the medication is safe and effective.9 However, not all medications require monitoring. For example, blood pressure readings may be sufficient to determine if the prescribed drug(s) are controlling hypertension. Furthermore, it may be difficult to monitor the target concentrations for certain drugs.
On the other hand, some medications have narrow therapeutic ranges and are known to cause adverse events or have marked pharmacokinetic variability. Additionally, drugs can be extremely expensive. These drugs would benefit from monitoring because the wrong drug or dosage can be a dangerous, costly mistake. The use of TDM helps create the best outcome for each patient by moving away from a “one size fits all” approach.
TDM for drugs and their corresponding ADA may be monitored using a variety of methods. Some commonly used technologies are ELISA, ChLIA, homogenous mobility shift assay (HMSA), LC-MS/MS, lateral flow assays, or cell-reporter assays. Samples tested are either serum or plasma. Unfortunately, international standards are not available for all drugs and ADA. Currently, there are no commercial proficiency testing schemes offered, thus preventing harmonization of results among different methodologies. It may be best to use the same assay to monitor drug concentration and ADA for a particular patient until harmonization is available.10-13 However, this could be problematic for patient care when an insurance plan dictates the use of a different laboratory or physician. There could be a shift in drug or ADA level, but the clinician will not know if this is due to differences in methodologies or actual physiological changes.
There are two main types of assays for ADA: drug sensitive (which measure free ADA) and drug tolerant (which measure both free ADA and those complexed to the corresponding drug, referred to as total ADA). Long-term outcomes are not adversely affected by the presence of total ADA in the absence of free ADA and the presence of adequate drug levels. Unfortunately, patients with free ADA and subtherapeutic drug levels typically have unfavorable outcomes.14
TDM is a step forward in personalized medicine for those suffering from chronic diseases. This field is everchanging as new drugs become available. It will be interesting to see the future impact of biologics and TDM on patient care.
References
1The Antibody Society. Therapeutic monoclonal antibodies approved or in review in the EU or US. (accessed Nov 16, 2023); www.antibodysociety.org/resources/approved-antibodies.
2 What Are “Biologics” Questions and Answers | FDA
3 Tumor Necrosis Factor Inhibitors – StatPearls – NCBI Bookshelf (nih.gov)
4E Zittan, B Kabakchiev, R Milgrom, G C Nguyen, K Croitoru, A H Steinhart, M S Silverberg. Higher Adalimumab Drug Levels are Associated with Mucosal Healing in Patients with Crohn’s Disease. J Crohns Colitis. 2016 May;10(5):510-5. doi: 10.1093/ecco-jcc/jjw014.
5N Vande Casteele ,M Ferrante , G Van Assche, V Ballet, G Compernolle, K Van Steen, S Simoens, P Rutgeerts, A Gils, S Vermeire. Gastroenterology. 2015 Jun;148(7):1320-9.e3. doi: 10.1053/j.gastro.2015.02.031.
6C Steenholdt, J Brynskov, O Østergaard Thomsen, L K Munck, J Fallingborg, L A Christensen, G Pedersen, J Kjeldsen, B A Jacobsen, A S Oxholm, J Kjellberg, K Bendtzen, M A Ainsworth. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014 Jun;63(6):919-27. doi: 10.1136/gutjnl-2013-305279.
7Thalayasingam N, Isaacs JD. Anti-TNF therapy. Best Pract Res Clin Rheumatol. 2011 Aug;25(4):549-67. doi: 10.1016/j.berh.2011.10.004
8Rosenblum H, Amital H. Anti-TNF therapy: safety aspects of taking the risk. Autoimmun Rev. 2011 Jul;10(9):563-8. doi: 10.1016/j.autrev.2011.04.010
9J-S Kang and M-H Lee. Overview of Therapeutic Drug Monitoring. Korean J Intern Med. 2009 Mar; 24(1): 1–10. doi: 10.3904/kjim.2009.24.1.1
10K Papamichael, W T Clarke, N Vande Casteele, K A Germansky, J D Feuerstein, GY Melmed, C A Siegel, P M Irving, A S Cheifetz. Comparison of Assays for Therapeutic Monitoring of Infliximab and Adalimumab in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol 2021 19:839-41.e2. doi: 10.1016/j.cgh.2020.03.002
11K Papamichael, V J Thomas, A Banty, W T Clarke, K A Germansky, S N Flier, J D Feuerstein, G Y Melmed, A S Cheifetz. Clinical Impact of Corrections to Infliximab and Adalimumab Monitoring Results with the Homogeneous Mobility Shift Assay. J Clin Med. 2020:9:2840-2849. doi.org/10.3390/jcm9092840
12F Silva-Ferreira, J Afonso, P Pinto-Lopes, F Magro. A Systematic Review on Infliximab and Adalimumab Drug Monitoring: Levels, Clinical Outcomes and Assays. Inflamm Bowel Dis 2016 Sep;22(9):2289-301. doi: 10.1097/MIB.0000000000000855
13D Jain, M T J Pido, J C Delgado, M A V Willrich, E Lázár-Molnár. Comparison of Two Clinical Laboratory Assays for Measuring Serum Adalimumab and Antibodies to Adalimumab. JALM; 8(06):1054-1064. doi:10.1093/jalm/jfad048
14Samaan M, Unsworth N, Ward M, B Warner, J Sanderson, Z Arkir, P Irving. PTH-074 The Presence of Total Anti-Drug Antibodies to Biologic Drugs Does not Adversely Affect Long-Term Outcomes in Crohn’s Disease Patients with Adequate Drugs Levels and Absent Free Anti-Drug Antibodies. Gut 2016;65:A255-A256. doi:10.1135/gutjnl-2016-312388.479